Abstract
Background/Introduction: The efficacy of Bcl-2 inhibitors in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) was established by the approval of agents such as venetoclax across all lines of therapy. However, adverse events (AEs) related to venetoclax and the development of BCL2 mutations leading to resistance may limit the utility of venetoclax in the clinic. Compared to venetoclax, BGB-11417 is a more potent (>10 fold in biochemical assays) and highly selective Bcl-2 inhibitor with the potential to achieve deeper target inhibition and clinical responses. Here, we report preliminary data from a phase 1 trial of BGB-11417 in patients with mature B-cell malignancies.
Methods: BGB-11417-102 (NCT04883957) is a multicenter, phase 1, open-label, dose-finding study conducted in China. The primary objectives were to determine the safety, maximum tolerated dose (MTD) or maximum administered dose, and the recommended phase 2 dose (RP2D) of BGB-11417 monotherapy for the selected B-cell malignancy dose-finding cohorts. Patients with relapsed/refractory (R/R) B-cell malignancies received escalating doses of BGB-11417 monotherapy (80, 160, 320, or 640 mg once daily) with a weekly or daily ramp-up to the intended target dose. Dose-limiting toxicity for each dose cohort was evaluated by a Bayesian logistic regression model during dose ramp-up through day 21 at intended dose. AEs were reported per Common Terminology Criteria for AEs v5.0.
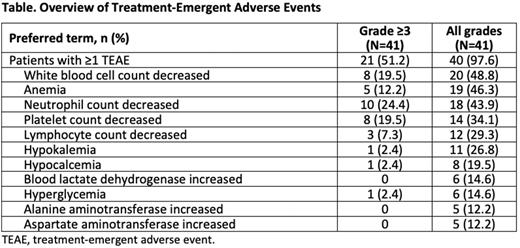
Results: As of June 16, 2022, 41 patients were treated. Twenty-seven with R/R non-Hodgkin lymphoma (NHL; 13 diffuse large B-cell lymphoma, 7 follicular lymphoma, 4 marginal zone lymphoma, and 3 transformed NHL) received BGB-11417 ≤640 mg/d and 14 with R/R CLL/SLL received ≤320 mg/d. MTD has not yet been reached. The median values were as follows: age, 62 years (range 31-84); lines of prior systemic therapy, 2 (range 1-7); and follow-up, 5.7 months (range 0.7-9.5). A total of 19 patients (18 NHL and 1 CLL/SLL) discontinued the study treatment (14 progressive disease, 4 withdrawal by subject, and 1 investigator decision). At least 1 treatment-emergent AE (TEAE) was reported by 97.6% of patients and grade ≥3 AEs were reported by 51.2%. The most common TEAEs (reported in ≥10% of patients) across all dose levels are listed in the Table. Dose-limiting toxicity events were reported in 7.3% of patients (grade 3 febrile neutropenia and grade 3 platelet count decrease at 80 mg/d dose level each; grade 3 bone pain at 160 mg/d dose level). Serious TEAEs were reported in 9.8% of patients, and TEAEs leading to drug interruption were reported in 12.2%. No patients reported TEAEs leading to death or treatment discontinuation. Laboratory tumor lysis syndrome (TLS) events were reported in 4.9% of patients, and all were controlled without dose modification. No clinical TLS events were reported. Of the 31 patients available for tumor assessment (23 NHL and 8 CLL/SLL), 4 patients with NHL and 5 patients with CLL/SLL achieved responses (NHL: 3 partial responses [PR] at 160 mg/d dose level and 1 PR at 320 mg/d dose level; CLL/SLL: 2 complete responses [CRs]/CR with incomplete count recovery, 1 PR at 80 mg/d dose level, and 2 PR at 160 mg/d dose level). One patient with CLL had a CLL count of less than 10-4 in both blood and bone marrow aspirate after 4.5 months of treatment with 80 mg/d dose, as measured by European Research Initiative in CLL flow cytometry assay with 10-4 sensitivity.
Conclusion: These results demonstrated that BGB-11417 is effective and well tolerated at all tested doses, with no increase in AEs with increasing dose. Dose escalation is still ongoing because an MTD has not yet been reached in any dose-escalation cohort as of the data cutoff date, with patients being recruited.
Disclosures
Zhang:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Shi:BeiGene, Ltd.: Current Employment, Current equity holder in publicly-traded company. Liang:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Wu:BeiGene: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.